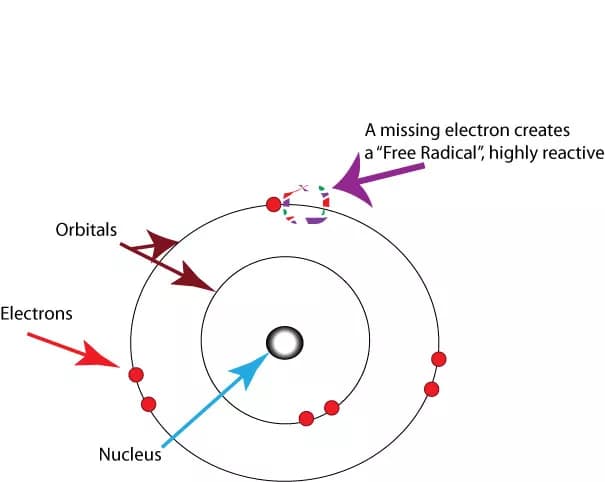
Free radicals are atoms or groups of atoms with an odd (unpaired) number of electrons. They can be formed when oxygen interacts with certain molecules. Oxygen is essential for human life, but may have life-threatening effects under certain circumstances. Many free radicals are unstable and very reactive. Once formed, these highly responsive radicals can start a chain reaction, like dominoes, and react with important cellular components such as DNA or the cell membrane. Other areas free radicals can attack are proteins, carbohydrates, and lipids, rendering them useless.
Free radicals can form from normal and essential metabolic process in the human body, or from external sources such as exposure to X-rays, ozone, cigarette smoking, air pollutants, and industrial chemicals. Normal cell functions manufacture a small percentage of free radicals, just like how a car engine emits fumes while it is running. These free radicals are usually not problematic; but those caused by the external toxins are what can cause cancers and damage to body parts.
Why are free radicals so harmful?
One of the major reasons why free radicals can be harmful is because it can cause a chain reaction. For example, if a free radical were to oxidize a fatty acid, it would change that fatty acid into a free radical, which then damages another fatty acid molecule.
When there is an unfavorable amount of free radical production, the tissue is under oxidative stress. Long-term oxidative stress has contribute to many conditions like atherosclerosis, certain cancers, and the process of aging. Other conditions that regular oxidative stresses also contribute to are inflammatory diseases (arthritis, vasculitis, and adult respiratory disease syndrome), ischemic diseases (heart diseases and stroke), acquired immunodeficiency syndrome (AIDS), stomach ulcers, high blood pressure, brain disorders (Alzheimer's disease, Parkinson's disease, and muscular dystrophy), alcoholism, and smoking-related diseases.
The body can be protected from many of these conditions by eating foods that are rich in antioxidants. Antioxidants are natural substances that may prevent or delay some types of oxidative cell damage by seeking and neutralizing free radicals.
Antioxidants are found in many foods including fruits and vegetables. Examples of antioxidant foods include the following:
- Beta-carotene
- Lutein
- Lycopene
- Selenium
- Vitamin A
- Vitamin C
- Vitamin E
How do antioxidants work?
There are two ways antioxidants in our body work. The first way is preventative antioxidants, which halt the formation of free radicals. The second is when the antioxidants scavenge the free radicals and helps prevent the occurrence of chain reactions. Nature provides thousands of different antioxidants in fruits, vegetables, whole grains, nuts, and legumes, which can eliminate free radicals. Adding more fruits and vegetables to the diet is a simple way to prevent oxidative stress and to keep one looking young.
Additional Resources:
Aruoma, O. I. (1994). Nutrition and health aspects of free radicals and antioxidants. Food and Chemical Toxicology, 32(7), 671-683.
Aruoma, O. I. (2003). Methodological considerations for characterizing potential antioxidant actions of bioactive components in plant foods. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis, 523, 9-20.
Cantuti-Castelvetri, I., Shukitt-Hale, B., & Joseph, J. A. (2000). Neurobehavioral aspects of antioxidants in aging. International Journal of Developmental Neuroscience, 18(4), 367-381.
Cheeseman, K. H., & Slater, T. F. (1993). An introduction to free radical biochemistry. British medical bulletin, 49(3), 481-493.
Halliwell, B. (1995, January). How to characterize an antioxidant: an update. InBiochem Soc Symp (Vol. 61, pp. 73-101).
Lobo, V., Patil, A., Phatak, A., & Chandra, N. (2010). Free radicals, antioxidants and functional foods: Impact on human health. Pharmacognosy reviews, 4(8), 118.
McCord, J. M. (2000). The evolution of free radicals and oxidative stress. The American journal of medicine, 108(8), 652-659.
Rice-Evans, C. A., & Diplock, A. T. (1993). Current status of antioxidant therapy. Free Radical Biology and Medicine, 15(1), 77-96.
Sokol, R. J. (1988). Vitamin E deficiency and neurologic disease. Annual review of nutrition, 8(1), 351-373.
Young, I. S., & Woodside, J. V. (2001). Antioxidants in health and disease.Journal of clinical pathology, 54(3), 176-186.
Helpful Peer-Reviewed Medical Articles:
Dröge, W. (2002). Free radicals in the physiological control of cell function.Physiological reviews, 82(1), 47-95.
Valko, M., Leibfritz, D., Moncol, J., Cronin, M. T., Mazur, M., & Telser, J. (2007). Free radicals and antioxidants in normal physiological functions and human disease. The international journal of biochemistry & cell biology,39(1), 44-84.
Valko, M., Rhodes, C. J., Moncol, J., Izakovic, M. M., & Mazur, M. (2006). Free radicals, metals and antioxidants in oxidative stress-induced cancer.Chemico-biological interactions, 160(1), 1-40.
Fang, Y. Z., Yang, S., & Wu, G. (2002). Free radicals, antioxidants, and nutrition. Nutrition, 18(10), 872-879.
Kehrer, J. P. (2008). Free radicals as mediators of tissue injury and disease.Critical reviews in toxicology.
McCord, J. M. (2000). The evolution of free radicals and oxidative stress.The American journal of medicine, 108(8), 652-659.
Related Articles
Test Your Knowledge
Asked by users
Related Centers
Related Specialties
Related Physicians
Related Procedures
Related Resources
Join DoveHubs
and connect with fellow professionals

0 Comments
Please log in to post a comment.