Mitochondrial Stress Induces Cancer-Related Metabolic Shifts
Cancerous tumors must be fed. Their unregulated growth requires a steady stream of blood flow and nutrients. Thus, one way that researchers have tried to wipe out cancer is to target cells undergoing the metabolic shifts that enable a tumor's rapid growth.
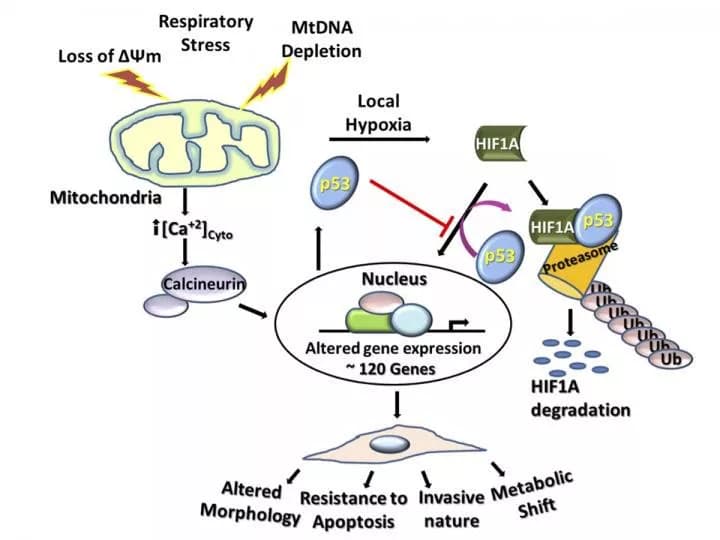
Yet new findings from University of Pennsylvania researchers suggest that such efforts might have missed a key pathway that enables the changes in metabolism that benefit tumors. Their work finds that mitochondrial stress alone can trigger metabolic shifts through a pathway that involves p53, a protein widely known to play multiple important roles in cancer.
"In all five cancer cell lines we looked at, we saw that p53 was induced when mitochondrial function was affected," said senior author Narayan Avadhani, the Harriet Ellison Woodward Professor of Biochemistry in Penn's School of Veterinary Medicine's Department of Biomedical Sciences. "This led to our discovery that it's possible to promote tumor growth independent of the HIF-1α pathway, which had up to this point been a prime target of therapeutic interventions."
The study points to a new factor that could inform our understanding of how cancer progresses. It's possible that markers of metabolic stress could even serve as a biomarker for a cancer's aggressiveness or likelihood to spread.
Avadhani teamed with Penn Vet's Anindya Roy Chowdhury, the lead author and a research associate, and Serge Y. Fuchs, professor of cell biology, as well as Ph.D. student Apple Long and Anil Rustgi, the T. Grier Miller Professor of Gastroenterology, both of Penn's Perelman School of Medicine. Avadhani and Fuchs are also members of Penn Vet's Mari Lowe Center for Comparative Oncology.
The work was published in the journal Oncogene.
In earlier studies, Avadhani and colleagues had shown that disrupting mitochondria could lead to tumor growth. Mitochondria are often referred to as the "powerhouses" of cells because they produce ATP, the molecular energy currency that cells utilize to perform their diverse functions. In related work, the researchers had also observed that subjecting mitochondria to stress also triggered an increase in p53 but, until now, hadn't conducted follow-up on that finding.
Because p53 is mutated in nearly 50 percent of human cancers, it is widely believed to have a tumor-suppressor function. The researchers decided to take a more detailed look into the connection between mitochondrial stress and p53.
They experimentally depleted mitochondrial DNA to induce mitochondrial stress in six cell lines, including several cancer cell lines, and found that p53 levels increased in response to the mtDNA depletion in each type of cell. Because HIF-1α activity is known to play both complementary and contradictory roles in cancer to p53, they next looked to see how that protein responded. They found that p53 inhibited HIF-1α activity.
Looking specifically at a human colon cancer cell line in which p53 was experimentally deleted, they again found a relationship with HIF-1α: Its activity was six-times higher in the colon cancer cell line with p53 depleted than in the wild type colon cancer cell line, a further indication that p53 inhibits HIF-1α.
To ensure that this was not strictly associated with depletion of mitochondrial DNA, the researchers induced mitochondrial stress using other means, including with chemicals agents and by disrupting the membrane, and found that all induced p53.
Further investigation revealed that p53 reduced HIF-1α levels in the nucleus and the cytoplasm of cells and that genes responsive to HIF-1α were blunted when mitochondrial DNA was depleted. Notably, they found that the expression of several genes involved with glycolysis, a metabolic process by which cells break down sugar to make energy, jumped dramatically in cells in which mtDNA was depleted. Some of these were the same genes that HIF-1α normally regulates, pointing to mitochondrial stress as a similar but completely separate pathway by which a metabolic shift can occur in cancer cells.
Finally, the team demonstrated that, in cells with depleted mtDNA, p53 physically interferes with HIF-1α by preventing it from binding to gene promoters that it would normally and by promoting ubiquitination of HIF-1α, a process that tags the protein for degradation in the cell.
The findings point to a new direction and possible new targets for preventing the metabolic shift that can foster a supportive environment for cancer growth.
"We show that mitochondrial stress is a force to be reckoned with," Avadhani said. "If people are only focused on HIF-1α to prevent a change in metabolism, that might not be enough. Mitochondrial stress can induce all those same changes."
Avadhani and colleagues are pursuing these leads, working to design therapeutic interventions that target the molecular markers of mitochondrial stress in an attempt to head off the metabolic shift that can help feed solid tumors.
The research was supported by the National Institutes of Health and Harriet Ellison Woodward Trust.
The above post is reprinted from materials provided by University of Pennsylvania. Note: Materials may be edited for content and length.
Disclaimer: DoveMed is not responsible for the adapted accuracy of news releases posted to DoveMed by contributing universities and institutions.
Primary Resource:
Chowdhury, A. R., Long, A., Fuchs, S. Y., Rustgi, A., & Avadhani, N. G. (2016). Mitochondrial stress-induced p53 attenuates HIF-1α activity by physical association and enhanced ubiquitination. Oncogene.
Related Articles
Test Your Knowledge
Asked by users
Related Centers
Related Specialties
Related Physicians
Related Procedures
Related Resources
Join DoveHubs
and connect with fellow professionals

0 Comments
Please log in to post a comment.