Breakthrough FDA Approvals Mark Milestone In Sickle Cell Treatment: CRISPR Therapy Takes Center Stage
In a groundbreaking move, the U.S. Food and Drug Administration (FDA) has granted approval for the first-ever gene-editing treatment for sickle cell disease, known as Casgevy. Developed through a collaboration between Vertex Pharmaceuticals and CRISPR Therapeutics, Casgevy employs the revolutionary CRISPR technology, recognized with a Nobel Prize in Chemistry, to edit human DNA and tackle the inherited blood disorder. This momentous decision, coming approximately a decade after the advent of CRISPR, is hailed as a significant stride in scientific achievement.
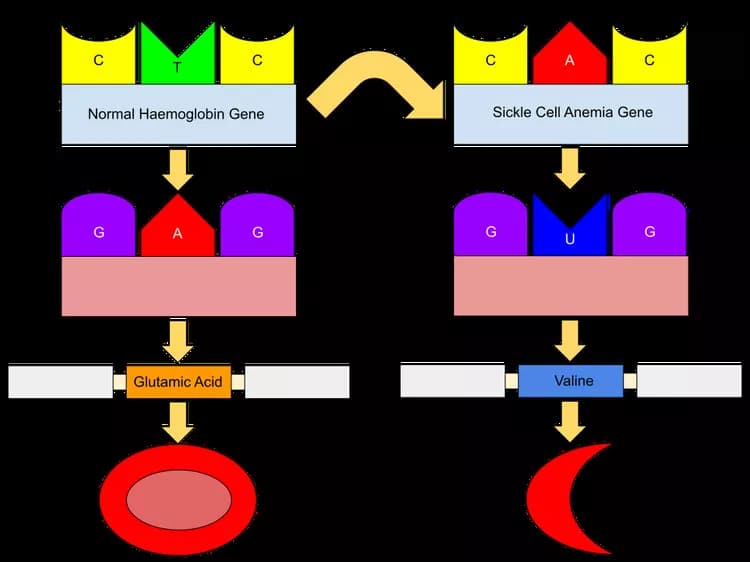
Sickle cell disease, affecting an estimated 100,000 Americans, leads to misshapen red blood cells causing painful blockages in blood vessels. Casgevy works by utilizing CRISPR to modify a patient's DNA, reactivating fetal hemoglobin that helps maintain the healthy shape of red blood cells. Clinical trials demonstrated remarkable success, eliminating pain crises in the majority of patients. The FDA has granted approval for Casgevy's use in individuals aged 12 and older.
While the therapy holds promise, the complexity of its administration poses challenges, including a multi-month process. Blood stem cells are extracted, genetically modified in Vertex's lab, and then reintroduced into patients after chemotherapy to replace old cells. Vertex, taking the lead on the drug's launch, plans to target the approximately 32,000 individuals in the U.S. and Europe with severe cases of sickle cell. However, concerns linger over the therapy's high cost, expected to be around $2 million per patient, and its limited accessibility to specialized health facilities.
In a parallel development, the FDA also approved a separate gene therapy, Lyfgenia by Bluebird Bio, offering an alternative approach to addressing sickle cell disease. This therapy, like Casgevy, targets patients aged 12 and older, aiming to eliminate pain crises.
The FDA's decision marks a pivotal moment in the treatment landscape for sickle cell disease, representing a significant advance in the realm of gene and cell-based therapies. Dr. Nicole Verdun, director of the FDA's Office of Therapeutic Products, emphasized the potential of gene therapy to provide more targeted and effective treatments, especially for rare diseases with limited treatment options.
Casgevy, formerly known as exagamglogene autotemcel, received conditional approval in the U.K. last month. The therapy's use of CRISPR/Cas9 technology represents a paradigm shift in gene editing for treating severe conditions, and its success opens the door to exploring similar approaches for other diseases. The FDA's commitment to facilitating the development of safe and effective treatments underscores the gravity of these approvals.
As Casgevy and Lyfgenia pave the way for innovative cell-based gene therapies, the broader implications of these FDA approvals extend beyond sickle cell disease. The stage is set for further exploration of CRISPR technology in treating various conditions, including cancer, diabetes, and A.L.S. This marks a crucial step in translating CRISPR from the research and development realm into clinical applications, offering hope to patients seeking transformative treatments for diseases with underlying genetic mutations.
Related Articles
Test Your Knowledge
Asked by users
Related Centers
Related Specialties
Related Physicians
Related Procedures
Related Resources
Join DoveHubs
and connect with fellow professionals

0 Comments
Please log in to post a comment.