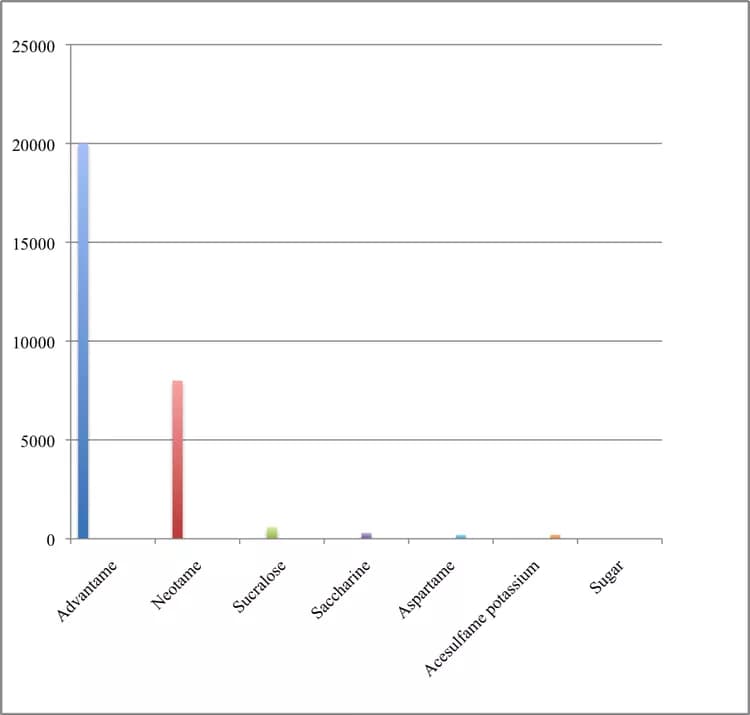
The U.S. Food and Drug Administration (FDA) approved a new sugar substitute called advantame on Monday (May 19th). Sugar substitutes duplicate the effect of sugar, but add hardly any or no calories to foods, and usually do not raise blood sugar levels.
“Sugar substitutes are called 'high-intensity' because small amounts pack a large punch when it comes to sweetness,” Captain Andrew Zajac, of the U.S. Public Health Service and director of the FDA's division of petition review, explained in the news release.
Advantame is a white powder that is approved for cooking, baked goods, non-alcoholic beverages including soft drinks, chewing gum, confections and frostings, frozen desserts, gelatins and puddings, jams and jellies, processed fruits and fruit juices, toppings, and syrups.
The new artificial sweetener is chemically similar to aspartame (Equal), and certain people should avoid or limit their use of aspartame, the FDA noted. These people have a genetic disorder called phenylketonuria (PKU), which makes it difficult for them to metabolize phenylalanine, an amino acid found in both aspartame and advantame. Food with aspartame must include label information warning people with PKU about the presence of phenylalanine.
Because advantame is much sweeter than aspartame, so a very small amount is needed, therefore, food containing advantame does not need special labeling, the FDA said.
Additional Resource:
Related Articles
Test Your Knowledge
Asked by users
Related Centers
Related Specialties
Related Physicians
Related Procedures
Related Resources
Join DoveHubs
and connect with fellow professionals

0 Comments
Please log in to post a comment.