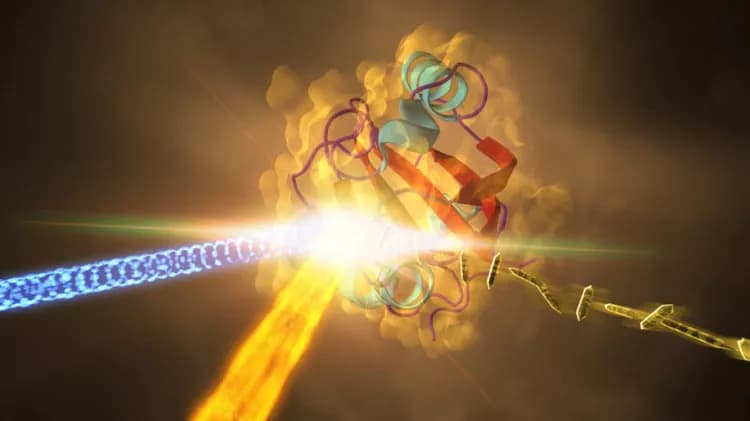
Fastest-Ever Molecular Imaging Reveals Reaction Crucial For Vision
Every process that sustains life is carried out by proteins, but understanding how these complex molecules do their jobs depends on learning the arrangement of their atoms -- and how this structure changes -- as they react. No imaging method for observing molecular movement in such detail and speed had been available, until now.
A team of biochemists and physicists, led by the University of Wisconsin-Milwaukee and Imperial College London, have documented for the first time the fundamental processes of a chemical reaction unfolding in real time. They captured images -- 25 trillion per second -- of a tiny crystalized protein as it reacted to light.
This allowed them build up a picture of what the protein was doing every few femtoseconds -- quadrillionths of a second. The results are published in the journal Science.
Previously, scientists had relied on a method called X-ray crystallography, which could only take a static image of a protein. Now, they have been able to build up a series of crystallographic snapshots into a molecular movie over extremely short timescales.
Study co-author Dr Jasper van Thor from Imperial's Department of Life Sciences said: "Usually, we can only image the structure after the reaction, and infer what has happened. This is the first time we have been able to image crystal structures on timescales where the proteins are still undergoing the reaction.
"What happens during these timescales determines the outcome of the reaction, so knowing exactly what's going on is vital. Previously our information and images of how the reactions work have been based on theory and spectroscopy. Now we can see it in reality."
The team studied a yellow dye molecule at the centre of a light-sensitive protein that undergoes a shape shift as it interacts with a photon, a particle of light. The basic biological process they observed is similar to how the human eye's retina responds to light.
Using the Linac Coherent Light Source X-ray Free Electron Laser in California, the team fired intensely bright pulses at the protein, snapping images every few femtoseconds as the photon reaction progressed. Crucially, the result was enabled by careful tailoring of the visible light pulse, which is necessary with very bright and very brief pulses.
Dr van Thor said: "We are working in interesting regimes that are new to crystallography, where the properties of the visible pulse matter most."
Next, the researchers will work on getting femtosecond details in order to actively control the dynamics. This could ultimately allow scientists to intervene in the process of protein functions by using light.
Now that the technique has been proven to work, the team hope it will start to be applied across molecular biology to unravel the mechanisms of all of proteins' crucial reactions for life.
"This puts us dramatically closer to understanding the chemistry necessary for all life," said Marius Schmidt, physics professor at UW-Milwaukee and co-author of the paper. "Discovering the step-by-step process of how proteins function is necessary not only to inform treatment of disease, but also to shed light on the grand questions of biology."
Imperial's Dr van Thor is investigating the areas where this technology could potentially lead to new breakthroughs, and is helping develop a case for a similar instrument facility to be built in the UK.
The above post is reprinted from materials provided by Imperial College London. The original item was written by Hayley Dunning. Note: Materials may be edited for content and length.
Disclaimer: DoveMed is not responsible for the adapted accuracy of news releases posted to DoveMed by contributing universities and institutions.
Primary Resource:
Pande, K., Hutchison, C. D., Groenhof, G., Aquila, A., Robinson, J. S., Tenboer, J., ... & White, T. A. (2016). Femtosecond structural dynamics drives the trans/cis isomerization in photoactive yellow protein. Science,352(6286), 725-729.
Related Articles
Test Your Knowledge
Asked by users
Related Centers
Related Specialties
Related Physicians
Related Procedures
Related Resources
Join DoveHubs
and connect with fellow professionals

0 Comments
Please log in to post a comment.